Publications
Publications in peer reviewed journals
Specific micropollutant biotransformation pattern by the comammox bacterium Nitrospira inopinata
2019 - Environ. Sci. Technol., 15: 8695-8705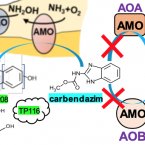
Abstract:
The recently discovered complete ammonia-oxidizing (comammox) bacteria occur in various environments, including wastewater treatment plants. To better understand their role in micropollutant biotransformation in comparison with ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA), we investigated the biotransformation capability of (the only comammox isolate) for 17 micropollutants. Asulam, fenhexamid, mianserin, and ranitidine were biotransformed by , (AOA), and Nm90 (AOB). More distinctively, carbendazim, a benzimidazole fungicide, was exclusively biotransformed by . The biotransformation of carbendazim only occurred when was supplied with ammonia but not nitrite as the energy source. The exclusive biotransformation of carbendazim by was likely enabled by an enhanced substrate promiscuity of its unique AMO and its much higher substrate (for ammonia) affinity compared with the other two ammonia oxidizers. One major plausible transformation product (TP) of carbendazim is a hydroxylated form at the aromatic ring, which is consistent with the function of AMO. These findings provide fundamental knowledge on the micropollutant degradation potential of a comammox bacterium to better understand the fate of micropollutants in nitrifying environments.
A Multicolor Fluorescence Hybridization Approach Using an Extended Set of Fluorophores to Visualize Microorganisms.
2019 - Front Microbiol, 10: 1383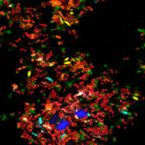
Abstract:
Fluorescence hybridization (FISH) with rRNA-targeted oligonucleotide probes is a key method for the detection of (uncultured) microorganisms in environmental and medical samples. A major limitation of standard FISH protocols, however, is the small number of phylogenetically distinct target organisms that can be detected simultaneously. In this study, we introduce a multicolor FISH approach that uses eight fluorophores with distinct spectral properties, which can unambiguously be distinguished by confocal laser scanning microscopy combined with white light laser technology. Hybridization of rRNA-targeted DNA oligonucleotide probes, which were mono-labeled with these fluorophores, to cultures confirmed that the fluorophores did not affect probe melting behavior. Application of the new multicolor FISH method enabled the differentiation of seven (potentially up to eight) phylogenetically distinct microbial populations in an artificial community of mixed pure cultures (five bacteria, one archaeon, and one yeast strain) and in activated sludge from a full-scale wastewater treatment plant. In contrast to previously published multicolor FISH approaches, this method does not rely on combinatorial labeling of the same microorganisms with different fluorophores, which is prone to biases. Furthermore, images acquired by this method do not require elaborate post-processing prior to analysis. We also demonstrate that the newly developed multicolor FISH method is compatible with an improved cell fixation protocol for FISH targeting Gram-negative bacterial populations. This fixation approach uses agarose embedding during formaldehyde fixation to better preserve the three-dimensional structure of spatially complex samples such as biofilms and activated sludge flocs. The new multicolor FISH approach should be highly suitable for studying structural and functional aspects of microbial communities in virtually all types of samples that can be analyzed by conventional FISH methods.
Global diversity and biogeography of bacterial communities in wastewater treatment plants.
2019 - Nat Microbiol, 7: 1183-1195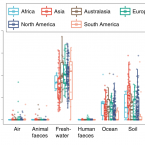
Abstract:
Microorganisms in wastewater treatment plants (WWTPs) are essential for water purification to protect public and environmental health. However, the diversity of microorganisms and the factors that control it are poorly understood. Using a systematic global-sampling effort, we analysed the 16S ribosomal RNA gene sequences from ~1,200 activated sludge samples taken from 269 WWTPs in 23 countries on 6 continents. Our analyses revealed that the global activated sludge bacterial communities contain ~1 billion bacterial phylotypes with a Poisson lognormal diversity distribution. Despite this high diversity, activated sludge has a small, global core bacterial community (n = 28 operational taxonomic units) that is strongly linked to activated sludge performance. Meta-analyses with global datasets associate the activated sludge microbiomes most closely to freshwater populations. In contrast to macroorganism diversity, activated sludge bacterial communities show no latitudinal gradient. Furthermore, their spatial turnover is scale-dependent and appears to be largely driven by stochastic processes (dispersal and drift), although deterministic factors (temperature and organic input) are also important. Our findings enhance our mechanistic understanding of the global diversity and biogeography of activated sludge bacterial communities within a theoretical ecology framework and have important implications for microbial ecology and wastewater treatment processes.
Cometabolic biotransformation and microbial-mediated abiotic transformation of sulfonamides by three ammonia oxidizers.
2019 - Water Res., 444-453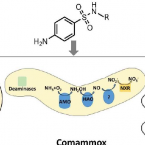
Abstract:
The abilities of three phylogenetically distant ammonia oxidizers, Nitrososphaera gargensis, an ammonia-oxidizing archaeon (AOA); Nitrosomomas nitrosa Nm90, an ammonia-oxidizing bacterium (AOB); and Nitrospira inopinata, the only complete ammonia oxidizer (comammox) available as a pure culture, to biotransform seven sulfonamides (SAs) were investigated. The removals and protein-normalized biotransformation rate constants indicated that the AOA strain N. gargensis exhibited the highest SA biotransformation rates, followed by N. inopinata and N. nitrosa Nm90. The transformation products (TPs) of sulfadiazine (SDZ), sulfamethazine (SMZ) and sulfamethoxazole (SMX) and the biotransformation mechanisms were evaluated. Based on the analysis of the TP formulas and approximate structures, it was found that during biotransformation, i) the AOA strain carried out SA deamination, hydroxylation, and nitration; ii) the AOB strain mainly performed SA deamination; and iii) the comammox isolate participated only in deamination reactions. It is proposed that deamination was catalyzed by deaminases while hydroxylation and nitration were mediated by nonspecific activities of the ammonia monooxygenase (AMO). Additionally, it was demonstrated that among the three ammonia oxidizers, only AOB contributed to the formation of pterin-SA conjugates. The biotransformation of SDZ, SMZ and SMX occurred only when ammonia oxidation was active, suggesting a cometabolic transformation mechanism. Interestingly, SAs could also be transformed by hydroxylamine, an intermediate of ammonia oxidation, suggesting that in addition to enzymatic conversions, a microbially induced abiotic mechanism contributes to SA transformation during ammonia oxidation. Overall, using experiments with pure cultures, this study provides important insights into the roles played by ammonia oxidizers in SA biotransformation.
Resolving the individual contribution of key microbial populations to enhanced biological phosphorus removal with Raman-FISH.
2019 - ISME J, 8: 1933-1946
Abstract:
Enhanced biological phosphorus removal (EBPR) is a globally important biotechnological process and relies on the massive accumulation of phosphate within special microorganisms. Candidatus Accumulibacter conform to the classical physiology model for polyphosphate accumulating organisms and are widely believed to be the most important player for the process in full-scale EBPR systems. However, it was impossible till now to quantify the contribution of specific microbial clades to EBPR. In this study, we have developed a new tool to directly link the identity of microbial cells to the absolute quantification of intracellular poly-P and other polymers under in situ conditions, and applied it to eight full-scale EBPR plants. Besides Ca. Accumulibacter, members of the genus Tetrasphaera were found to be important microbes for P accumulation, and in six plants they were the most important. As these Tetrasphaera cells did not exhibit the classical phenotype of poly-P accumulating microbes, our entire understanding of the microbiology of the EBPR process has to be revised. Furthermore, our new single-cell approach can now also be applied to quantify storage polymer dynamics in individual populations in situ in other ecosystems and might become a valuable tool for many environmental microbiologists.