Publications
Publications in peer reviewed journals
Visualisation of the obligate hydrocarbonoclastic bacteria Polycyclovorans algicola and Algiphilus aromaticivorans in co-cultures with micro-algae by CARD-FISH.
2018 - J. Microbiol. Methods, 73-79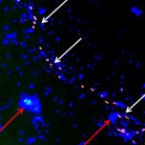
Abstract:
Some studies have described the isolation and 16S rRNA gene sequence-based identification of hydrocarbon-degrading bacteria living associated with marine eukaryotic phytoplankton, and thus far the direct visual observation of these bacteria on micro-algal cell surfaces ('phycosphere') has not yet been reported. Here, we developed two new 16S rRNA-targeted oligonucleotide probes, PCY223 and ALGAR209, to respectively detect and enumerate the obligate hydrocarbonoclastic bacteria Polycyclovorans algicola and Algiphilus aromaticivorans by Catalyzed Reporter Deposition Fluorescence in situ Hybridization (CARD-FISH). To enhance the hybridization specificity with the ALGAR209 probe, a competitor probe was developed. These probes were tested and optimized using pure cultures, and then used in enrichment experiments with laboratory cultures of micro-algae exposed to phenanthrene, and with coastal water enriched with crude oil. Microscopic analysis revealed these bacteria are found in culture with the micro-algal cells, some of which were found attached to algal cells, and whose abundance increased after phenanthrene or crude oil enrichment. These new probes are a valuable tool for identifying and studying the ecology of P. algicola and A. aromaticivorans in laboratory and field samples of micro-algae, as well as opening new fields of research that could harness their ability to enhance the bioremediation of contaminated sites.
Microbial nitrogen limitation in the mammalian large intestine.
2018 - Nat Microbiol, 12: 1441-1450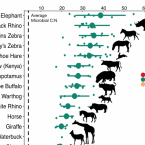
Abstract:
Resource limitation is a fundamental factor governing the composition and function of ecological communities. However, the role of resource supply in structuring the intestinal microbiome has not been established and represents a challenge for mammals that rely on microbial symbionts for digestion: too little supply might starve the microbiome while too much might starve the host. We present evidence that microbiota occupy a habitat that is limited in total nitrogen supply within the large intestines of 30 mammal species. Lowering dietary protein levels in mice reduced their faecal concentrations of bacteria. A gradient of stoichiometry along the length of the gut was consistent with the hypothesis that intestinal nitrogen limitation results from host absorption of dietary nutrients. Nitrogen availability is also likely to be shaped by host-microbe interactions: levels of host-secreted nitrogen were altered in germ-free mice and when bacterial loads were reduced via experimental antibiotic treatment. Single-cell spectrometry revealed that members of the phylum Bacteroidetes consumed nitrogen in the large intestine more readily than other commensal taxa did. Our findings support a model where nitrogen limitation arises from preferential host use of dietary nutrients. We speculate that this resource limitation could enable hosts to regulate microbial communities in the large intestine. Commensal microbiota may have adapted to nitrogen-limited settings, suggesting one reason why excess dietary protein has been associated with degraded gut-microbial ecosystems.
Stable-isotope probing of human and animal microbiome function
2018 - Trends Microbiol, 26: 999-1007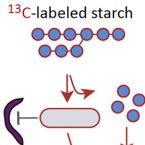
Abstract:
Humans and animals host diverse communities of microorganisms important to their physiology and health. Despite extensive sequencing-based characterization of host-associated microbiomes, there remains a dramatic lack of understanding of microbial functions. Stable-isotope probing (SIP) is a powerful strategy to elucidate the ecophysiology of microorganisms in complex host-associated microbiotas. Here, we suggest that SIP methodologies should be more frequently exploited as part of a holistic functional microbiomics approach. We provide examples of how SIP has been used to study host-associated microbes in vivo and ex vivo. We highlight recent developments in SIP technologies and discuss future directions that will facilitate deeper insights into the function of human and animal microbiomes.
Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass
2018 - Science Advances, 4: eaas9024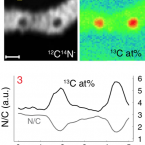
Abstract:
Plastic materials are widely used in agricultural applications to achieve food security for the growing world population. The use of biodegradable instead of nonbiodegradable polymers in single-use agricultural applications, including plastic mulching, promises to reduce plastic accumulation in the environment. We present a novel approach that allows tracking of carbon from biodegradable polymers into CO2 and microbial biomass. The approach is based on 13C-labeled polymers and on isotope-specific analytical methods, including nanoscale secondary ion mass spectrometry (NanoSIMS). Our results unequivocally demonstrate the biodegradability of poly(butylene adipate-co-terephthalate) (PBAT), an important polyester used in agriculture, in soil. Carbon from each monomer unit of PBAT was used by soil microorganisms, including filamentous fungi, to gain energy and to form biomass. This work advances both our conceptual understanding of polymer biodegradation and the methodological capabilities to assess this process in natural and engineered environments.
Signatures of ecological processes in microbial community time series.
2018 - Microbiome, 1: 120
Abstract:
Growth rates, interactions between community members, stochasticity, and immigration are important drivers of microbial community dynamics. In sequencing data analysis, such as network construction and community model parameterization, we make implicit assumptions about the nature of these drivers and thereby restrict model outcome. Despite apparent risk of methodological bias, the validity of the assumptions is rarely tested, as comprehensive procedures are lacking. Here, we propose a classification scheme to determine the processes that gave rise to the observed time series and to enable better model selection.
We implemented a three-step classification scheme in R that first determines whether dependence between successive time steps (temporal structure) is present in the time series and then assesses with a recently developed neutrality test whether interactions between species are required for the dynamics. If the first and second tests confirm the presence of temporal structure and interactions, then parameters for interaction models are estimated. To quantify the importance of temporal structure, we compute the noise-type profile of the community, which ranges from black in case of strong dependency to white in the absence of any dependency. We applied this scheme to simulated time series generated with the Dirichlet-multinomial (DM) distribution, Hubbell's neutral model, the generalized Lotka-Volterra model and its discrete variant (the Ricker model), and a self-organized instability model, as well as to human stool microbiota time series. The noise-type profiles for all but DM data clearly indicated distinctive structures. The neutrality test correctly classified all but DM and neutral time series as non-neutral. The procedure reliably identified time series for which interaction inference was suitable. Both tests were required, as we demonstrated that all structured time series, including those generated with the neutral model, achieved a moderate to high goodness of fit to the Ricker model.
We present a fast and robust scheme to classify community structure and to assess the prevalence of interactions directly from microbial time series data. The procedure not only serves to determine ecological drivers of microbial dynamics, but also to guide selection of appropriate community models for prediction and follow-up analysis.Fluorinated Gold Nanoparticles for Nanostructure Imaging Mass Spectrometry.
2018 - ACS Nano, 7: 6938-6948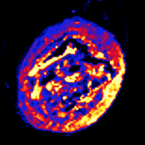
Abstract:
Nanostructure imaging mass spectrometry (NIMS) with fluorinated gold nanoparticles (f-AuNPs) is a nanoparticle assisted laser desorption/ionization approach that requires low laser energy and has demonstrated high sensitivity. Here we describe NIMS with f-AuNPs for the comprehensive analysis of metabolites in biological tissues. F-AuNPs assist in desorption/ionization by laser-induced release of the fluorocarbon chains with minimal background noise. Since the energy barrier required to release the fluorocarbons from the AuNPs is minimal, the energy of the laser is maintained in the low μJ/pulse range, thus limiting metabolite in-source fragmentation. Electron microscopy analysis of tissue samples after f-AuNP NIMS shows a distinct "raising" of the surface as compared to matrix assisted laser desorption ionization ablation, indicative of a gentle desorption mechanism aiding in the generation of intact molecular ions. Moreover, the use of perfluorohexane to distribute the f-AuNPs on the tissue creates a hydrophobic environment minimizing metabolite solubilization and spatial dislocation. The transfer of the energy from the incident laser to the analytes through the release of the fluorocarbon chains similarly enhances the desorption/ionization of metabolites of different chemical nature, resulting in heterogeneous metabolome coverage. We performed the approach in a comparative study of the colon of mice exposed to three different diets. F-AuNP NIMS allows the direct detection of carbohydrates, lipids, bile acids, sulfur metabolites, amino acids, nucleotide precursors as well as other small molecules of varied biological origins. Ultimately, the diversified molecular coverage obtained provides a broad picture of a tissue's metabolic organization.
Recognizing Patterns: Spatial Analysis of Observed Microbial Colonization on Root Surfaces
2018 - Front Environ Sci, 6: 1-12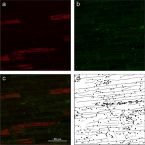
Abstract:
Root surfaces are major sites of interactions between plants and associated microorganisms. Here, plants and microbes communicate via signaling molecules, compete for nutrients, and release substrates that may have beneficial or harmful effects on each other. Whilst the body of knowledge on the abundance and diversity of microbial communities at root-soil interfaces is now substantial, information on their spatial distribution at the microscale is still scarce.
In this study, a standardized method for recognizing and analyzing microbial cell distributions on root surfaces is presented. Fluorescence microscopy was combined with automated image analysis and spatial statistics to explore the distribution of bacterial colonization patterns on rhizoplanes of rice roots. To test and evaluate the presented approach, a gnotobiotic experiment was performed using a potential nitrogen-fixing bacterial strain in combination with roots of wetland rice.
The automated analysis procedure resulted in reliable spatial data of bacterial cells colonizing the rhizoplane. Among all replicate roots, the analysis revealed an increasing density of bacterial cells from the root tip to the region of root cell maturation. Moreover, bacterial cells showed significant spatial clustering and tended to be located around plant root cell walls. The quantitative data suggest that the structure of the root surface plays a major role in bacterial colonization patterns.
Possible adaptations of the presented approach for future studies are discussed along with potential pitfalls such as inaccurate imaging. Our results demonstrate that standardized recognition and statistical evaluation of microbial colonization on root surfaces holds the potential to increase our understanding of microbial associations with roots and of the underlying ecological interactions.Bacterial interactions during sequential degradation of cyanobacterial necromass in a sulfidic arctic marine sediment
2018 - Environ Microbiol, 20: 2927–2940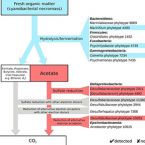
Abstract:
Seafloor microorganisms impact global carbon cycling by mineralizing vast quantities of organic matter (OM) from pelagic primary production, which is predicted to increase in the Arctic because of diminishing sea ice cover. We studied microbial interspecies-carbon-flow during anaerobic OM degradation in arctic marine sediment using stable isotope probing. We supplemented sediment incubations with 13C-labeled cyanobacterial necromass (spirulina), mimicking fresh OM input, or acetate, an important OM degradation intermediate, and monitored sulfate reduction rates and concentrations of volatile fatty acids (VFAs) during substrate degradation. Sequential 16S rRNA gene and transcript amplicon sequencing and fluorescence in situ hybridization combined with Raman microspectroscopy revealed that only few bacterial species were the main degraders of 13C-spirulina necromass. Psychrilyobacter, Psychromonas, Marinifilum, Colwellia, Marinilabiaceae and Clostridiales species were likely involved in the primary hydrolysis and fermentation of spirulina. VFAs, mainly acetate, produced from spirulina degradation were mineralized by sulfate-reducing bacteria and an Arcobacter species. Cellular activity of Desulfobacteraceae and Desulfobulbaceae species during acetoclastic sulfate reduction was largely decoupled from relative 16S rRNA gene abundance shifts. Our findings provide new insights into the identities and physiological constraints that determine the population dynamics of key microorganisms during complex OM degradation in arctic marine sediments.
Long-distance electron transport in individual, living cable bacteria.
2018 - Proc. Natl. Acad. Sci. U.S.A., 22: 5786-5791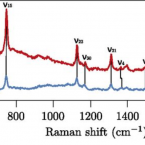
Abstract:
Electron transport within living cells is essential for energy conservation in all respiring and photosynthetic organisms. While a few bacteria transport electrons over micrometer distances to their surroundings, filaments of cable bacteria are hypothesized to conduct electric currents over centimeter distances. We used resonance Raman microscopy to analyze cytochrome redox states in living cable bacteria. Cable-bacteria filaments were placed in microscope chambers with sulfide as electron source and oxygen as electron sink at opposite ends. Along individual filaments a gradient in cytochrome redox potential was detected, which immediately broke down upon removal of oxygen or laser cutting of the filaments. Without access to oxygen, a rapid shift toward more reduced cytochromes was observed, as electrons were no longer drained from the filament but accumulated in the cellular cytochromes. These results provide direct evidence for long-distance electron transport in living multicellular bacteria.
Evaluation of primers targeting the diazotroph functional gene and development of NifMAP – a bioinformatics pipeline for analyzing nifH amplicon data
2018 - Front Microbiol, 9: 1-15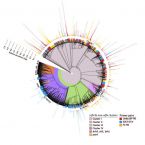
Abstract:
Diazotrophic microorganisms introduce biologically available nitrogen (N) to the global N cycle through the activity of the nitrogenase enzyme. The genetically conserved dinitrogenase reductase (nifH) gene is phylogenetically distributed across four clusters (I-IV) and is widely used as a marker gene for N2 fixation, permitting investigators to study the genetic diversity of diazotrophs in nature and target potential participants in N2 fixation. To date there have been limited, standardized pipelines for the nifH functional gene, which is in stark contrast to the rRNA gene. Here we present a bioinformatics pipeline for processing nifH amplicon datasets – NifMAP (“NifH MiSeq Illumina amplicon Analysis Pipeline”), which as a novel aspect uses Hidden-Markov models to filter out homologous genes to nifH. By using this pipeline, we evaluated the broadly inclusive primer pairs (Ueda19F-R6, IGK3-DVV, F2-R6) that target the nifH gene. To evaluate any systematic biases, the nifH gene was amplified with the aforementioned primer pairs in a diverse collection of environmental samples (soils, rhizosphere and roots samples, biological soil crusts and estuarine samples), in addition to a nifH mock community consisting of six phylogenetically diverse members. We noted that all primer pairs co-amplified nifH homologs to varying degrees; up to 90% of the amplicons were nifH homologs with IGK3-DVV in some samples (rhizosphere and roots from tall oat-grass). In regards to specificity, we observed some degree of bias across the primer pairs. For example, primer pair F2-R6 discriminated against cyanobacteria (amongst others), yet captured many sequences from subclusters IIIE and IIIL-N. These aforementioned subclusters were largely missing by the primer pair IGK3-DVV, which also tended to discriminate against Alphaproteobacteria, but amplified sequences within clusters IIIC (affiliated with Clostridia) and clusters IVB and IVC. Primer pair Ueda19F-R6 exhibited the least bias and successfully captured diazotrophs in cluster I and subclusters IIIE, IIIL, IIIM and IIIN, but discriminated against Firmicutes and subcluster IIIC. Taken together, our newly established bioinformatics pipeline, NifMAP, along with our systematic evaluations of nifH primer pairs permit more robust, high-throughput investigations of diazotrophs in diverse environments.
Application of stable-isotope labelling techniques for the detection of active diazotrophs
2018 - Environmental Microbiology, 20: 44-61
Abstract:
Investigating active participants in the fixation of dinitrogen gas is vital as N is often a limiting factor for primary production. Biological nitrogen fixation (BNF) is performed by a diverse guild of bacteria and archaea (diazotrophs), which can be free-living or symbionts. Free-living diazotrophs are widely distributed in the environment, yet our knowledge about their identity and ecophysiology is still limited. A major challenge in investigating this guild is inferring activity from genetic data as this process is highly regulated. To address this challenge, we evaluated and improved several 15N-based methods for detecting N2 fixation activity (with a focus on soil samples) and studying active diazotrophs. We compared the acetylene reduction assay and the 15N2 tracer method and demonstrated that the latter is more sensitive in samples with low activity. Additionally, tracing 15N into microbial RNA provides much higher sensitivity compared to bulk soil analysis. Active soil diazotrophs were identified with a 15N-RNA-SIP approach optimized for environmental samples and benchmarked to 15N-DNA-SIP. Lastly, we investigated the feasibility of using SIP-Raman microspectroscopy for detecting 15N-labelled cells. Taken together, these tools allow identifying and investigating active free-living diazotrophs in a highly sensitive manner in diverse environments, from bulk to the single-cell level.
NanoSIMS and tissue autoradiography reveal symbiont carbon fixation and organic carbon transfer to giant ciliate host.
2018 - ISME J, 3: 714-727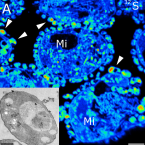
Abstract:
The giant colonial ciliate Zoothamnium niveum harbors a monolayer of the gammaproteobacteria Cand. Thiobios zoothamnicoli on its outer surface. Cultivation experiments revealed maximal growth and survival under steady flow of high oxygen and low sulfide concentrations. We aimed at directly demonstrating the sulfur-oxidizing, chemoautotrophic nature of the symbionts and at investigating putative carbon transfer from the symbiont to the ciliate host. We performed pulse-chase incubations with C- and C-labeled bicarbonate under varying environmental conditions. A combination of tissue autoradiography and nanoscale secondary ion mass spectrometry coupled with transmission electron microscopy was used to follow the fate of the radioactive and stable isotopes of carbon, respectively. We show that symbiont cells fix substantial amounts of inorganic carbon in the presence of sulfide, but also (to a lesser degree) in the absence of sulfide by utilizing internally stored sulfur. Isotope labeling patterns point to translocation of organic carbon to the host through both release of these compounds and digestion of symbiont cells. The latter mechanism is also supported by ultracytochemical detection of acid phosphatase in lysosomes and in food vacuoles of ciliate cells. Fluorescence in situ hybridization of freshly collected ciliates revealed that the vast majority of ingested microbial cells were ectosymbionts.