Publications
Publications in peer reviewed journals
Bacterial interactions during sequential degradation of cyanobacterial necromass in a sulfidic arctic marine sediment
2018 - Environ Microbiol, 20: 2927–2940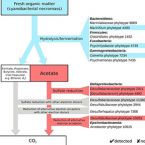
Abstract:
Seafloor microorganisms impact global carbon cycling by mineralizing vast quantities of organic matter (OM) from pelagic primary production, which is predicted to increase in the Arctic because of diminishing sea ice cover. We studied microbial interspecies-carbon-flow during anaerobic OM degradation in arctic marine sediment using stable isotope probing. We supplemented sediment incubations with 13C-labeled cyanobacterial necromass (spirulina), mimicking fresh OM input, or acetate, an important OM degradation intermediate, and monitored sulfate reduction rates and concentrations of volatile fatty acids (VFAs) during substrate degradation. Sequential 16S rRNA gene and transcript amplicon sequencing and fluorescence in situ hybridization combined with Raman microspectroscopy revealed that only few bacterial species were the main degraders of 13C-spirulina necromass. Psychrilyobacter, Psychromonas, Marinifilum, Colwellia, Marinilabiaceae and Clostridiales species were likely involved in the primary hydrolysis and fermentation of spirulina. VFAs, mainly acetate, produced from spirulina degradation were mineralized by sulfate-reducing bacteria and an Arcobacter species. Cellular activity of Desulfobacteraceae and Desulfobulbaceae species during acetoclastic sulfate reduction was largely decoupled from relative 16S rRNA gene abundance shifts. Our findings provide new insights into the identities and physiological constraints that determine the population dynamics of key microorganisms during complex OM degradation in arctic marine sediments.
Metaproteogenomic Profiling of Microbial Communities Colonizing Actively Venting Hydrothermal Chimneys.
2018 - Front Microbiol, 680
Abstract:
At hydrothermal vent sites, chimneys consisting of sulfides, sulfates, and oxides are formed upon contact of reduced hydrothermal fluids with oxygenated seawater. The walls and surfaces of these chimneys are an important habitat for vent-associated microorganisms. We used community proteogenomics to investigate and compare the composition, metabolic potential and relative protein abundance of microbial communities colonizing two actively venting hydrothermal chimneys from the Manus Basin back-arc spreading center (Papua New Guinea). We identified overlaps in the functional profiles of both chimneys, despite differences in microbial community composition and venting regime. Carbon fixation on both chimneys seems to have been primarily mediated through the reverse tricarboxylic acid cycle and fueled by sulfur-oxidation, while the abundant metabolic potential for hydrogen oxidation and carbon fixation via the Calvin-Benson-Bassham cycle was hardly utilized. Notably, the highly diverse microbial community colonizing the analyzed black smoker chimney had a highly redundant metabolic potential. In contrast, the considerably less diverse community colonizing the diffusely venting chimney displayed a higher metabolic versatility. An increased diversity on the phylogenetic level is thus not directly linked to an increased metabolic diversity in microbial communities that colonize hydrothermal chimneys.
Peatland Acidobacteria with a dissimilatory sulfur metabolism
2018 - ISME J, 12: 1729-1742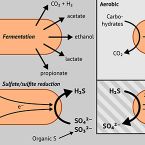
Abstract:
Sulfur-cycling microorganisms impact organic matter decomposition in wetlands and consequently greenhouse gas emissions from these globally relevant environments. However, their identities and physiological properties are largely unknown. By applying a functional metagenomics approach to an acidic peatland, we recovered draft genomes of seven novel Acidobacteria species with the potential for dissimilatory sulfite (dsrAB, dsrC, dsrD, dsrN, dsrT, dsrMKJOP) or sulfate respiration (sat, aprBA, qmoABC plus dsr genes). Surprisingly, the genomes also encoded DsrL, which so far was only found in sulfur-oxidizing microorganisms. Metatranscriptome analysis demonstrated expression of acidobacterial sulfur-metabolism genes in native peat soil and their upregulation in diverse anoxic microcosms. This indicated an active sulfate respiration pathway, which, however, might also operate in reverse for dissimilatory sulfur oxidation or disproportionation as proposed for the sulfur-oxidizing Desulfurivibrio alkaliphilus. Acidobacteria that only harbored genes for sulfite reduction additionally encoded enzymes that liberate sulfite from organosulfonates, which suggested organic sulfur compounds as complementary energy sources. Further metabolic potentials included polysaccharide hydrolysis and sugar utilization, aerobic respiration, several fermentative capabilities, and hydrogen oxidation. Our findings extend both, the known physiological and genetic properties of Acidobacteria and the known taxonomic diversity of microorganisms with a DsrAB-based sulfur metabolism, and highlight new fundamental niches for facultative anaerobic Acidobacteria in wetlands based on exploitation of inorganic and organic sulfur molecules for energy conservation.
Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle
2018 - ISME J, 12: 1715-1728
Abstract:
A critical step in the biogeochemical cycle of sulfur on Earth is microbial sulfate reduction, yet organisms from relatively few lineages have been implicated in this process. Previous studies using functional marker genes have detected abundant, novel dissimilatory sulfite reductases (DsrAB) that could confer the capacity for microbial sulfite/sulfate reduction but were not affiliated with known organisms. Thus, the identity of a significant fraction of sulfate/sulfite-reducing microbes has remained elusive. Here we report the discovery of the capacity for sulfate/sulfite reduction in the genomes of organisms from thirteen bacterial and archaeal phyla, thereby more than doubling the number of microbial phyla associated with this process. Eight of the thirteen newly identified groups are candidate phyla that lack isolated representatives, a finding only possible given genomes from metagenomes. Organisms from Verrucomicrobia and two candidate phyla, Candidatus Rokubacteria and Candidatus Hydrothermarchaeota, contain some of the earliest evolved dsrAB genes. The capacity for sulfite reduction has been laterally transferred in multiple events within some phyla, and a key gene potentially capable of modulating sulfur metabolism in associated cells has been acquired by putatively symbiotic bacteria. We conclude that current functional predictions based on phylogeny significantly underestimate the extent of sulfate/sulfite reduction across Earth’s ecosystems. Understanding the prevalence of this capacity is integral to interpreting the carbon cycle because sulfate reduction is often coupled to turnover of buried organic carbon. Our findings expand the diversity of microbial groups associated with sulfur transformations in the environment and motivate revision of biogeochemical process models based on microbial community composition.
Draft genome sequence of Telmatospirillum siberiense 26-4b1T, an acidotolerant peatland alphaproteobacterium potentially involved in sulfur cycling
2018 - Genome Announc, 6: e01524-17
Abstract:
The facultative anaerobic chemoorganoheterotrophic alphaproteobacterium Telmatospirillum siberiense 26-4b1T was isolated from a Siberian peatland. We report on a 6.20 Mbp near complete, high quality draft genome of T. siberiense that reveals expected and novel metabolic potential for the genus Telmatospirillum, including genes for sulfur oxidation.
NanoSIMS and tissue autoradiography reveal symbiont carbon fixation and organic carbon transfer to giant ciliate host.
2018 - ISME J, 3: 714-727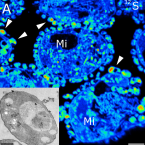
Abstract:
The giant colonial ciliate Zoothamnium niveum harbors a monolayer of the gammaproteobacteria Cand. Thiobios zoothamnicoli on its outer surface. Cultivation experiments revealed maximal growth and survival under steady flow of high oxygen and low sulfide concentrations. We aimed at directly demonstrating the sulfur-oxidizing, chemoautotrophic nature of the symbionts and at investigating putative carbon transfer from the symbiont to the ciliate host. We performed pulse-chase incubations with C- and C-labeled bicarbonate under varying environmental conditions. A combination of tissue autoradiography and nanoscale secondary ion mass spectrometry coupled with transmission electron microscopy was used to follow the fate of the radioactive and stable isotopes of carbon, respectively. We show that symbiont cells fix substantial amounts of inorganic carbon in the presence of sulfide, but also (to a lesser degree) in the absence of sulfide by utilizing internally stored sulfur. Isotope labeling patterns point to translocation of organic carbon to the host through both release of these compounds and digestion of symbiont cells. The latter mechanism is also supported by ultracytochemical detection of acid phosphatase in lysosomes and in food vacuoles of ciliate cells. Fluorescence in situ hybridization of freshly collected ciliates revealed that the vast majority of ingested microbial cells were ectosymbionts.
Evidence for H2 consumption by uncultured Desulfobacterales in coastal sediments.
2018 - Environ. Microbiol., 2: 450-461
Abstract:
Molecular hydrogen (H ) is the key intermediate in the anaerobic degradation of organic matter. Its removal by H -oxidizing microorganisms is essential to keep anaerobic degradation energetically favourable. Sulfate-reducing microorganisms (SRM) are known as the main H scavengers in anoxic marine sediments. Although the community of marine SRM has been extensively studied, those consuming H in situ are completely unknown. We combined metagenomics, PCR-based clone libraries, single-amplified genomes (SAGs) and metatranscriptomics to identify potentially H -consuming SRM in anoxic coastal sediments. The vast majority of SRM-related H ase sequences were assigned to group 1b and 1c [NiFe]-H ases of the deltaproteobacterial order Desulfobacterales. Surprisingly, the same sequence types were similarly highly expressed in spring and summer, suggesting that these are stable and integral members of the H -consuming community. Notably, one sequence cluster from the SRM group 1 consistently accounted for around half of all [NiFe]-H ase transcripts. Using SAGs, we could link this cluster with the 16S rRNA genes of the uncultured Sva0081-group of the family Desulfobacteraceae. Sequencing of 16S rRNA gene amplicons and H ase gene libraries suggested consistently high in situ abundance of the Sva0081 group also in other marine sediments. Together with other Desulfobacterales these likely are important H -scavengers in marine sediments.